The exchange capacity of aquarium substrates is very often mentioned in discussions between aquarists, in aquarium substrate sheets and in various other cases.
What does this mean?
Let’s see!
Exchange capacity
Substrates and soils contain net (i.e. non-equilibrium) electrical charges.
Depending on the type of resulting net charge – positive or negative – we will have different exchange capacities.

Cation exchange capacity (CSC – CEC)
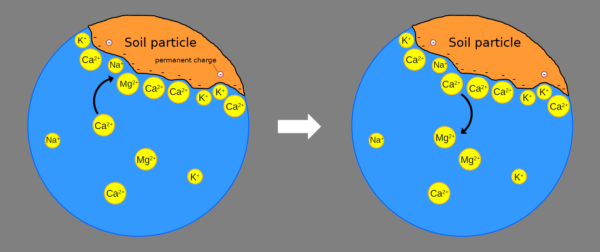
Substrates containing particles known as aluminosilicates, i.e. crystals composed of aluminium (Al) and silicon (Si), or organic matter, have a predominantly negative electrical charge, which attracts positively charged particles, also known as cations.
There are basically two reasons for the presence of these negative charges:
- breakage of the outer layers of the silicates that make them up, with exposure of the “edges”, which are usually negatively charged;
- substitution within the structure of the silicates by certain cations, such as Mg2+, of other more charged cations, such as Al3+.
These negative charges, as we mentioned earlier, are able to attract positively charged ions (cations), such as the ammonium ion NH4+ or calcium and magnesium ions (Ca2+ and K+).
Cations are not attracted randomly: they are attracted with different strengths, depending on valence and other parameters (hydrated ionic radius etc) and those present in greater quantities are attracted more.
For example, some of the nutrients bind in this order:
H+ ≥ Al3+ > Ca2+ > Mg2+ > NH4+ = K+ > Na
H = hydrogen, Al = aluminium, Ca = calcium, Mg = magnesium, NH4+ = ammonium, K = potassium, Na = sodium
… where on the left are the most easily exchanged elements.
This series is a type of lyotropic series.
Similarly, some transition metals tend to follow this order:
Cu2+ > Ni2+ > Fe2+ > Mn2+
Cu = copper, Ni = nickel, Fe = iron, Mn = manganese
How is the exchange capacity measured?
The total amount of negative charge in a substrate is called the cation exchange capacity (CSC).
The CSC is commonly measured in milliequivalents [meq] per unit weight; one meq is equivalent to 6×1020 (6 followed by 20 zeros) negative charges.
Below are some typical values for some substrates. To give a yardstick, in agriculture a soil with a CSC of more than 15-20 meq/100 grams is considered to have a good exchange capacity:
- sand 3-5 meq/100 grams
- clay soil 10-15 meq/100 grams
- silt 15-25 meq/100 grams
- clay 20-50 meq/100 grams
- zeolite 200-400 meq/100 grams
- humus 300-500 meq/100 grams (decreases with increasing pH)
To give an example in words, a substrate with a CSC of 10 meq/100 grams has 60 followed by twenty zeros negative charges per 100 grams!
Anion exchange capacity (CSA – AEC)
Contrary to what we have seen so far, different types of substrate, containing aluminium and silicon in amorphous (non-crystallised) form and iron or aluminium oxides, have a predominantly positive electrical charge, capable of attracting negatively charged particles, also known as anions.
Examples of anions are the sulphate ion (SO42-), the nitrate ion (NO3–), the phosphate ion (PO43-), the chloride ion (Cl–) or the hydrogen carbonate ion (HCO3–, bicarbonate).
The behaviour is quite similar to that of cations, only the sign of the charge changes (negative, instead of positive).
Along the lines of the affinity order of cations, the binding order of some anions is as follows:
NO3– < Cl– < SO42- < PO43-
Nitrates < Chlorides < Sulphates < Phosphates
i.e. nitrates are weakly bound, whereas phosphates are more strongly bound (and the exchange is therefore more burdensome).
The anion exchange capacity is always measured in meq/quantity of substrate or soil, similar to the CSC.
In reality, the anion exchange capacity is generally lower than the cation exchange capacity, so that only the latter is often mentioned. However, (at least) both are present and the anion exchange capacity is generally sufficient for the substrate to retain some anions, such as sulphates or phosphates.
Exchange capacity and aquarium plants
What is the point of all this discussion about exchange capacity?
When a substrate has a high exchange capacity it would be intuitive to think that it binds nutrients to itself and does not make them available to plants.
In reality, the forces of attraction between substrate and nutrients are relatively weak, allowing an exchange between the nutrients in the water, the nutrients on the surface of the substrate particles and the plant roots.
How a substrate facilitates nutrient uptake
When the roots of the plants move through the substrate, they either encounter the nutrient directly and take it up or they have to wait for it to arrive somehow.
A substrate with a good exchange capacity facilitates this work: the substrate has various nutrients loosely bound to it, which the plants can absorb and exchange more easily than fetching them from the water.
Taking up dissolved nutrient ions from the water, on the other hand, requires much more energy from the plants and some of them may not be able to do this at all.
How to manage a bottom with exchange capacity in an aquarium?
Many aquarium substrates have exchange capacity, such as fertile substrates with an organic or clay component or so-called allophanic substrates (a rather inappropriate term, as allophanic may be a component of these substrates but is practically never the only one), such as some ADA, Elos or Prodibio substrates or the common Akadama (bonsai soil), to name but a few.

Initial release and uptake
These bottoms tend to absorb nutrients as soon as they are placed in the aquarium, lowering measurable levels in the water.
It is common, for example, to observe significant decreases in carbonate hardness (absorption of carbonates), phosphates or iron.
Depending on the specific composition of the substrate, we may also see releases; iron and nitrate releases are not uncommon.
For example, ADA bottoms tend to release ammonium or nitrate initially; conversely, some batches of Akadama release iron.
The fact is that the substrate absorbs cations and anions from the water column and binds them to the particles in it.
If there was already some element being exchanged, it may be that by order of affinity this will be ‘displaced’ by other elements, so we have the releases measured above, in addition to the absorptions.
Fertilisation management
In the first period after set up, fertilisation must be carried out with particular care, both because the “active” bottom alters the expected values in the column as a result of its exchanges, and because the aquarium has just started, the plants have just been introduced and the various balances (chemical, biological, etc.) have yet to form.
It will therefore be necessary to take due account of what is being added and to observe the response of the plants, in addition to the test results (which can be misleading in these cases).
For example, many substrates tend to zero phosphates in the water: if we only looked at the tests, we would have to put a lot back in immediately.
However, phosphates are bound to the substrate and plants can take them up. Sometimes it can happen that the substrate releases, so if we have an increase in phosphate in an aquarium without having added it and we had previously measured an uptake, it is most likely that this phosphate is returned from the substrate.
Nutrient storage capability
It is also good to remember that these bottoms have a sort of nutrient storage capacity, but should not be bombarded with nutrients to saturate them quickly: the risk of sudden releases is high and risky. It is better to proceed with proper fertilisation over time, observing the plants and their possible signs of deficiencies or problems.
Finally, as far as durability is concerned, with time and the attainment of various balances the bottom will apparently stop exchanging, so we will no longer measure sharp drops in carbonates, phosphates or ammonium releases, just to name a few common phenomena.
This does not mean, however, that the soil has lost its exchange capacity, quite the contrary: a good balance between exchanged elements has been achieved.
This is different for so-called fertile soils, which lose their fertility over time as plants absorb the fertilisers they contain (iron, potassium, trace elements…). However, the component of these fertile grounds with exchange capacity – if present – retains its properties: if necessary, the fertiliser component can be replenished using sticks, tabs or fertilising spheres.
Credits
CSC diagram: By Kyle MoJo – Own work, CC0, https://commons.wikimedia.org/w/index.php?curid=62069074
Allophane: By Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=15277038